TELDAP Collections
| Electron Diffraction |
|
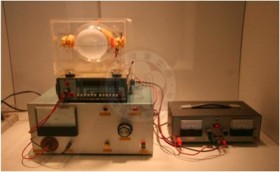
The purpose of this experiment is to calculate the electron wave length and verify the hypothesis of Louis De Broglie, proposed in 1924 by the Fench physicist Louis De Broglie, who received the Nobel Prize in Physics in 1929. He considered that both electrons and other particles exhibit the wave properties, and the wave length was determined by the momentum of the particle. This experiment uses high voltage source to accelerate electrons by 2500~5000 volts to have the electrons injected to a graphite crystal and to observe the diffraction pattern on the screen.
Electron Diffraction Tube
Video Electron Diffraction
|